Integrated Structural Biology seeks to unravel the complexity of biological systems by combining structural knowledge at multiple resolution levels within their cellular contexts, while incorporating temporal dynamics. No single technique—whether X-ray diffraction, NMR, or electron microscopy—can capture the full spectrum of structural, dynamical, and functional properties of macromolecules. Consequently, the integration of biophysical methods, supported by computational modeling, has become essential for the study of complex biological pathways. Technological advances now allow us to probe systems previously inaccessible to conventional methods and to characterize transient interactions both inside and outside the cell.
NMR spectroscopy plays a crucial role in the characterization of difficult systems such as largely unstructured proteins, transient protein complexes, fibrils, and protein embedded in matrices.
Solution NMR is an indispensable enabling technology for determining weak and transient macromolecule interactions as well as for characterizing functional processes in solution and also directly in living cells. CERM applies Solution NMR in an integrated structural biology approach for addressing more and more challenging questions. Such approach is for example routinely used to understand cellular pathways at the atomic level. Solution NMR is also largely applied in CERM to reveal the presence of conformational states in solution and to monitor conformational rearrangements in systems composed of multiple domains.
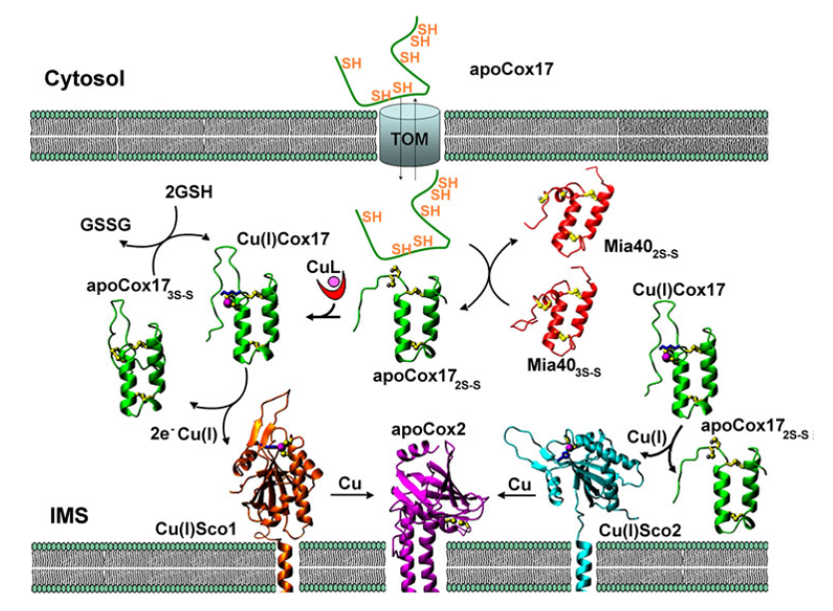
Integrated structural biology approach to unravel copper trafficking in the mitochondrion to assembly CuA site of cytochrome c oxidase. ref: Cell Mol Life Sci. 2010 Aug;67(15):2563-89. e Acc Chem Res. 2018 Jun 19;51(6):1550-1557.
Solid-state NMR (ssNMR), plays a central role in this integrated approach, providing high-resolution insights into challenging systems such as high molecular weight proteins, transient protein complexes, fibrils, and proteins embedded in heterogeneous matrices. Recent advances in solid-state NMR (SSNMR) have significantly improved sensitivity and resolution. Ultrafast magic angle spinning (MAS) up to 110 kHz and ultra-high magnetic fields, as high as 28.2 T, now allow to record high-resolution ¹H detected spectra, greatly increasing sensitivity and the possible applications compared to conventional heteronuclear detection. SSNMR finds direct applications in pressing biomedical challenges, such as antimicrobial resistance, a leading cause of mortality that demands new antibiotics.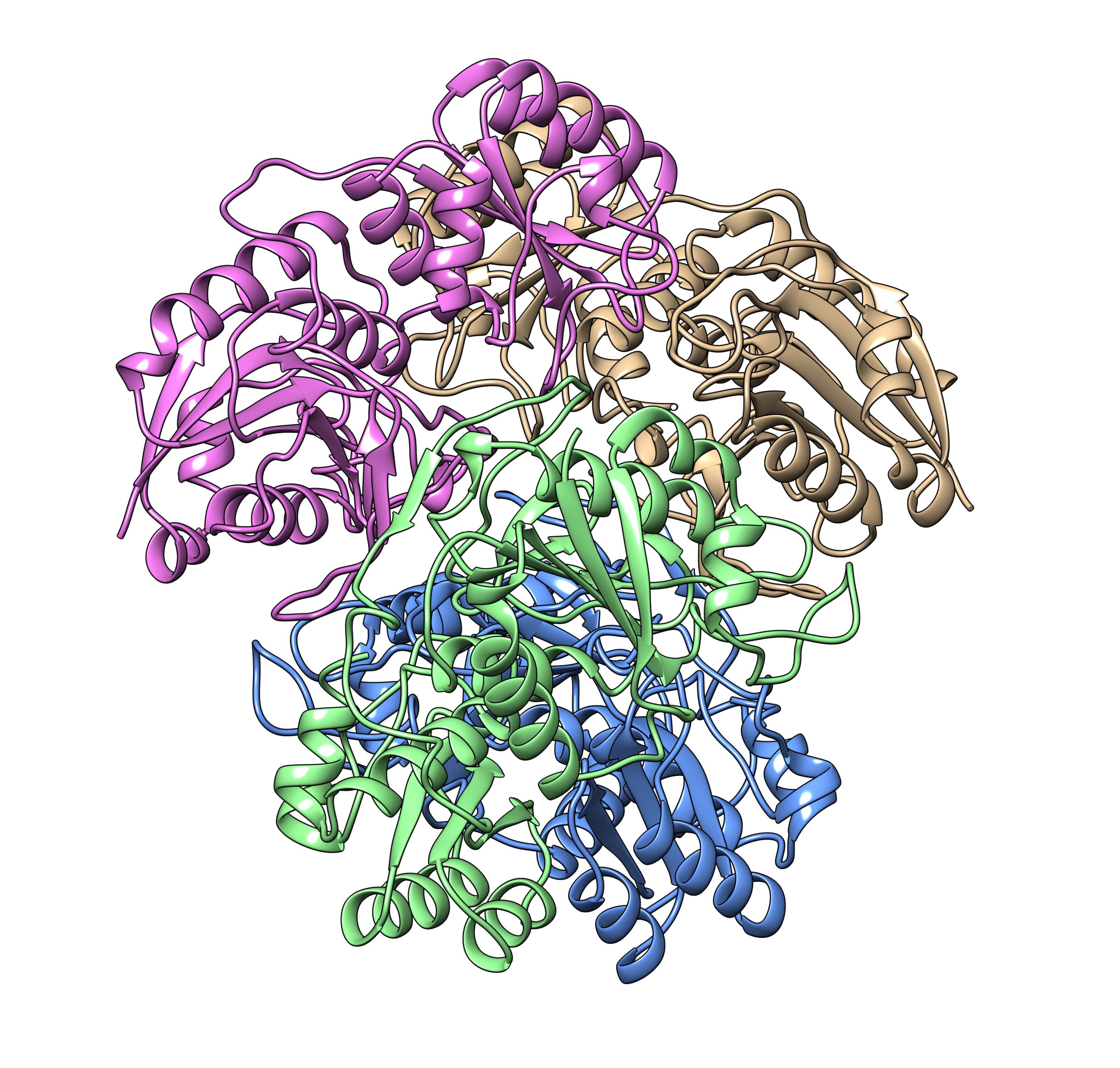
Structural model of the tetramer of the pegylated asparaginase protein (Chemistry. 2019 Feb 6; 25 (8): 1984-1991. doi: 10.1002 / chem.201804488) with experimental constraints obtained from the analysis of solid-state NMR spectra.
The power of Integrated Structural Biology is further amplified by combining NMR with complementary biophysical techniques available at CERM/CIRMMP, including fluorescence spectroscopy, circular dichroism, isothermal titration calorimetry, SEC/FFF-MALS, and stopped-flow experiments. These combined approaches allow precise characterization of protein folding, conformational transitions, and transient interactions that govern regulatory mechanisms. The synergy between experimental and computational methods enhances the reliability of kinetic data and deepens understanding of allosteric modulation and transient binding events, ultimately providing a comprehensive framework for decoding the intricate dynamics of biological systems. Recent publications from our scientists illustrate how this integrated strategy can reveal molecular mechanisms underlying antibiotic action, photoreceptor adaptation, and pharmaceutical interactions.


