In-cell NMR provides unique details on the structure, dynamics and function of macromolecules inside living cells at atomic resolution. At CERM, we have developed a protein expression approach to observe proteins in human cells by NMR, which allows studying protein functional processes, such as folding, maturation and interactions, in their native environment. The highly physiologically relevant data obtained complement the structural characterization usually carried out in vitro, and allow elucidating important phenomena such as protein-drug interactions.
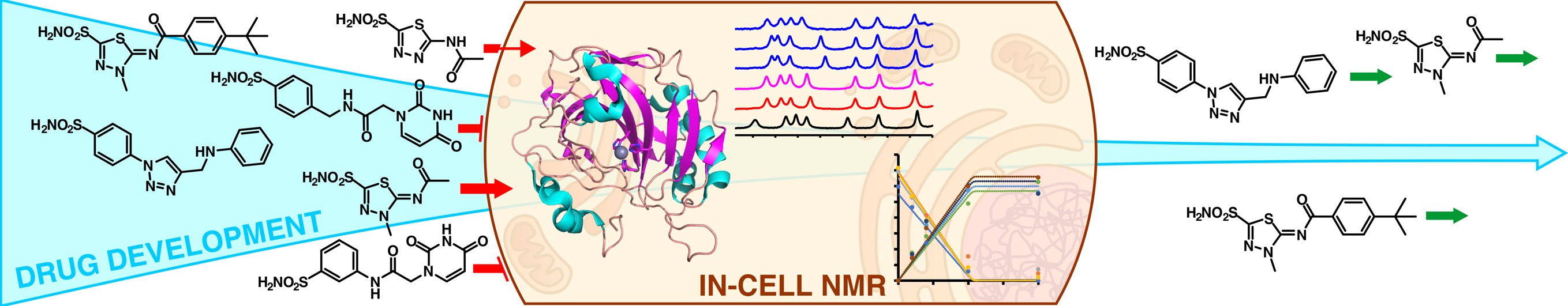
A flow NMR bioreactor has been developed at CERM that greatly extends the lifetime of the cells up to several days. With this setup, protein conformational changes and drug binding can be monitored in real time, allowing both the structural characterization of the protein-ligand complex and the assessment of membrane permeability and binding selectivity towards the target, which are critical parameters to improve the potency of potential drugs.
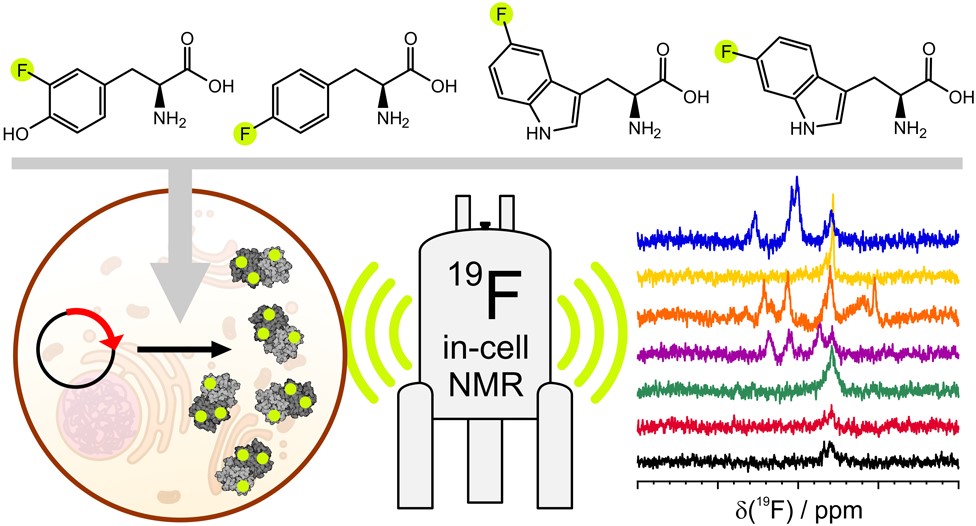
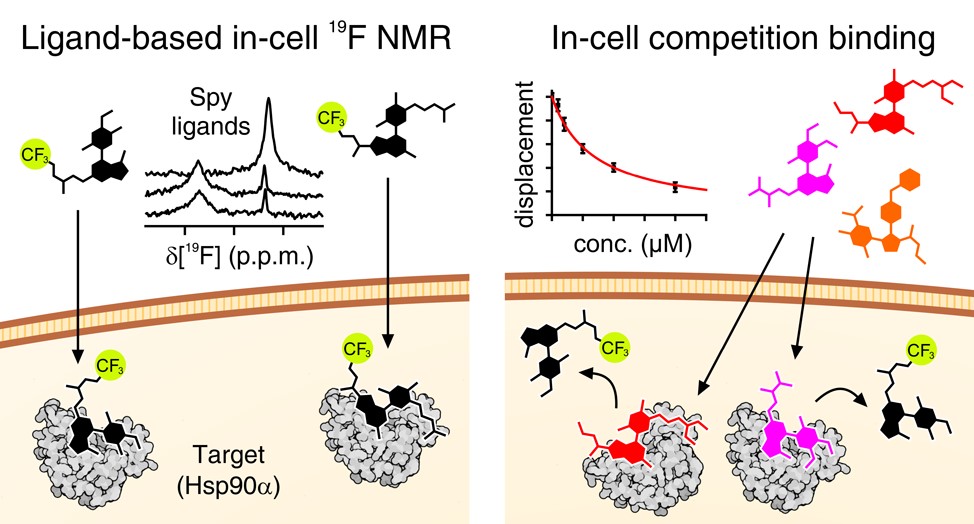
Advanced labelling schemes which exploit the sensitive, background-free nature of 19F have recently been developed. 19F NMR allows both protein and ligand observation, providing a unique way to study the kinetics of ligand binding to otherwise ‘invisible’ intracellular target proteins.
Novel labelling strategies are being established to achieve cost-effective selective side-chain labelling of proteins expressed in human cells, extending the range of in-cell NMR applications to increasingly challenging proteins.
In parallel, CERM has advanced in-cell EPR methods to investigate the behavior of spin labels in cellular environments.Recent studies compared relaxation properties in protonated versus deuterated cells, providing insights into the optimal conditions for EPR measurements inside living cells.
(1) Luchinat, E., Cremonini, M., & Banci, L. Radio signals from live cells: the coming of age of in-cell solution NMR. Chemical reviews, 2022, 122(10), 9267-9306. https://doi.org/10.1021/acs.chemrev.1c00790
(2) Luchinat, E., Barbieri, L., Cremonini, M., Nocentini, A., Supuran, C. T., & Banci, L. Drug Screening in Human Cells by NMR Spectroscopy Allows the Early Assessment of Drug Potency. Angewandte Chemie International Edition, 2020, 59(16), 6535-6539. https://doi.org/10.1002/anie.201913436
(3) Luchinat, E., Barbieri, L., Campbell, T. F., & Banci, L. Real-time quantitative in-cell NMR: ligand binding and protein oxidation monitored in human cells using multivariate curve resolution. Analytical chemistry, 2020, 92(14), 9997-10006. https://doi.org/10.1021/acs.analchem.0c01677
(4) Luchinat, E., Barbieri, L., Cremonini, M., Pennestri, M., Nocentini, A., Supuran, C. T., & Banci, L. Determination of intracellular protein–ligand binding affinity by competition binding in-cell NMR. Acta Crystallographica. Section D, Structural Biology, 2021, 77(Pt 10), 1270. https://doi.org/10.1107/S2059798321009037
(5) Pham, L. B., Costantino, A., Barbieri, L., Calderone, V., Luchinat, E., & Banci, L. Direct expression of fluorinated proteins in human cells for 19F in-cell NMR spectroscopy. Journal of the American Chemical Society, 2023, 145(2), 1389-1399. https://doi.org/10.1021/jacs.2c12086
(6) Luchinat, E., Barbieri, L., Davis, B., Brough, P. A., Pennestri, M., & Banci, L. Ligand-Based Competition Binding by Real-Time 19F NMR in Human Cells. Journal of Medicinal Chemistry, 2024, 67(2), 1115-1126. https://doi.org/10.1021/acs.jmedchem.3c01600
(7) Rosati, M., Barbieri, L., Hlavac, M., Kratzwald, S., Lichtenecker, R. J., Konrat, R., Luchinat, E. & Banci, L. Towards cost-effective side-chain isotope labelling of proteins expressed in human cells. Journal of Biomolecular NMR, 2024, 78, 237-247. https://doi.org/10.1007/s10858-024-00447-6
(8) Torricella, F., Vitali, V., Banci, L. A systematic study on the effect of protonation and deuteration on electron spin Tₘ/T₂ in a cellular context. Phys. Chem. Chem. Phys., 2024, 26, 20246–20250. https://doi.org/10.1039/D4CP00599F

