Structural protein stability within the cell as a new Achilles heel to tackle antibiotic resistance
A MAECI-MinCyt joint project between CERM (Florence, Italy) and IBR (Rosario, Argentina)
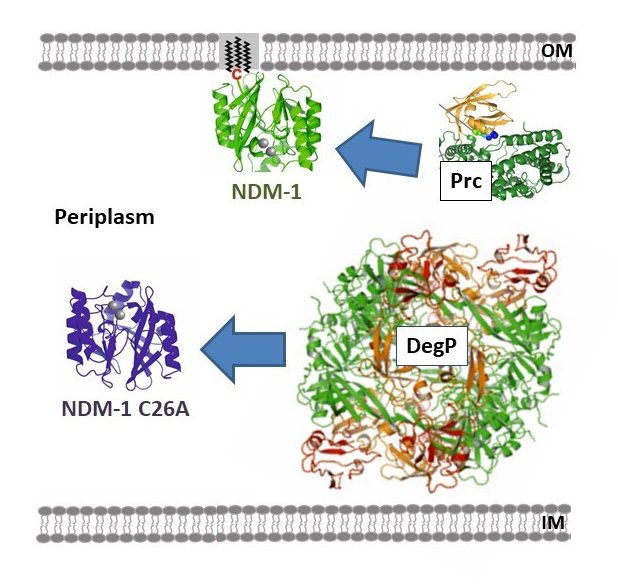 Antibiotic resistance is nowadays one of the most serious public health threats. This problem is rapidly accelerating due to the worldwide spread of antibiotic-resistance genes, leaving few options for clinical treatment. Within this context Carbapenems are last resort b-lactam antibiotics. However, carbapenems are inactivated by zinc-dependent metallo-b-lactamases (MBLs), such as Bacillus cereus II (BcII) and New Delhi metallo-b-lactamase (NDM-1), present in multi-resistant bacterial pathogens. The host innate immune elicits a response to bacterial infection that involves sequestering of essential metal ions, such as Zn(II), from the pathogens. We have recently shown that this response triggers the degradation of MBLs within bacteria, rendering them susceptible to antibiotics. Different MBLs display distinct stabilities upon metal restriction: while soluble NDM-1 is readily degraded, soluble BcII and membrane anchored NDM-1 are refractory to this process. In this project we seek to understand the intracellular metabolic fate of two paradigmatic lactamases (BcII and NDM-1) under conditions of variable Zn(II) availability. To this aim, we will exploit novel in-cell NMR techniques and microbiology tools to unravel the folding and Zn(II) uptake mechanisms of MBLs within the bacterial cell. In addition, we will characterize how metal ion deprivation results in enzyme degradation and how membrane anchoring modulates protein stability.
Antibiotic resistance is nowadays one of the most serious public health threats. This problem is rapidly accelerating due to the worldwide spread of antibiotic-resistance genes, leaving few options for clinical treatment. Within this context Carbapenems are last resort b-lactam antibiotics. However, carbapenems are inactivated by zinc-dependent metallo-b-lactamases (MBLs), such as Bacillus cereus II (BcII) and New Delhi metallo-b-lactamase (NDM-1), present in multi-resistant bacterial pathogens. The host innate immune elicits a response to bacterial infection that involves sequestering of essential metal ions, such as Zn(II), from the pathogens. We have recently shown that this response triggers the degradation of MBLs within bacteria, rendering them susceptible to antibiotics. Different MBLs display distinct stabilities upon metal restriction: while soluble NDM-1 is readily degraded, soluble BcII and membrane anchored NDM-1 are refractory to this process. In this project we seek to understand the intracellular metabolic fate of two paradigmatic lactamases (BcII and NDM-1) under conditions of variable Zn(II) availability. To this aim, we will exploit novel in-cell NMR techniques and microbiology tools to unravel the folding and Zn(II) uptake mechanisms of MBLs within the bacterial cell. In addition, we will characterize how metal ion deprivation results in enzyme degradation and how membrane anchoring modulates protein stability.
The project is funded by 
