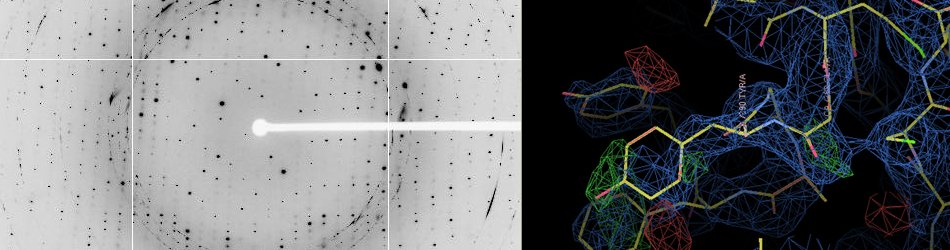
X-Ray Crystallography
According to the Protein Data Bank, X-ray crystallography is by far the most used technique (141654 over 159140 entries as of December 31st 2019), ultimately allowing the determination of the atomic structure of bio-macromolecules.
X-ray diffraction beamlines at modern synchrotron radiation facilities have played a pivotal role in modern macromolecular crystallography. Due to the design of dedicated high-intensity and bright X-ray diffraction beamlines, beam sizes and crystal dimensions are on the micrometer scale and it is possible to collect a complete data set in seconds, and obtain the resulting structure in minutes.
High-throughput X-ray crystallography is of the greatest importance in drug discovery where the process of solving ligand-bound protein structures include time-consuming screenings of pharmaceutical lead compounds. Moreover, the advantage of rapid data collection is apparent when exploring libraries of chemical compounds for fragment-based drug screening.
More recently, Serial Milliseconds Crystallography (SMX), is emerging as an interesting alternative to conventional data collection where a continuous flux of microcrystals, nano-micrometer in size (avoiding the need of well-formed protein crystals) is injected at room temperature into the X-ray beam path with a time resolution in the range of milliseconds.
X-ray Scattering in Solution
Small-angle X-ray scattering (SAXS) is a biophysical method to study the overall shape and structural transitions of biological macromolecules in solution. SAXS provides low-resolution (1-2 nm) information on the shape, conformation and assembly state of proteins, nucleic acids and various macromolecular complexes. The technique also offers powerful means for the quantitative analysis of flexible systems, including intrinsically disordered proteins. The resolution and reliability of SAXS-based structural models, using high intensity X-rays available at dedicated beamlines at synchrotron, have increased significantly over the past decade. Subsequently, biological SAXS revealed a rapid growth of popularity in the scientific community worldwide. The synergistic application of SAXS with high-resolution methods like X-ray crystallography and Nuclear Magnetic Resonance (NMR), as well as in silico long timescale atomistic simulations, provides an extremely useful approach that facilitates comprehensive characterization of structural ensembles of flexibly linked multi-domain systems and of conformational transitions associated to functional activities.
Fiber X-ray Diffraction
Small (SAXS) and Wide angle X-ray scattering (WAXS) can be used simultaneously to inspect hierarchically ordered materials, such as fibers. The favorable combination of the two techniques allows to inspect sub-molecular (WAXS) and supramolecular (SAXS) organization of the fibers, which are known to order at the different length scales. Fibers can be studied in solution or as solid materials. In case of solid materials the possibility to collect SAXS/WAXS data with a micro-source may add the possibility to study the material across a third length scale given by the X-ray probe size. In this way micro, nano and atomic scales can be probe at the same time, within the same experiment.
